|
|||||||
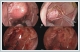
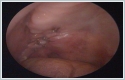
AbstractTumors of parapharyngeal space (PPS) are uncommon and mostly benign which generally originate from salivary and neurogenic tissues. Salivary gland tumors, most of which are pleomorphic adenoma, predominate in pre-styloid space. Complete resection of the tumors is the primary treatment. Because of its location in the deep neck space, a pre-styloid tumor can be resected via transoral approach. The use of rigid endoscopes in this approach provides satisfactory exposure and safe surgery. In this report, we present a case of 67-year-old male with a large, encapsulated, pre-styloid PPS tumor who underwent endoscope-assisted transoral excision. Postoperatively, the patient had no complications and his swallowing and chewing were not affected.IntroductionThe parapharyngeal space (PPS) is an inverted pyramid-shaped anatomical region extending from the skull base to the hyoid bone. This region is divided into two compartments, as the pre-styloid and the post-styloid space. Tumors of PPS are uncommon and account for 0.5% - 1.5% of all head and neck tumors, among which the frequency of benign tumors is approximately 80%.[1,2] Most tumors in the pre-styloid space originate from salivary gland tissues. The most common pathologic type is pleomorphic adenoma (PA).[3] Surgery is the mainstay of treatment of PSS tumors. However, surgical treatment of PPS tumors is challenging due to the deep localization of this space and its close relationship with vital structures in this region and any damage to this structures can be life threatening.[4] Various surgical approaches had been described. External approaches, including the transcervical, transparotid and transmandibular approaches, are most commonly used in the PPS tumors.[5] Although these approaches provide wide surgical exposure, they have more complications, cosmetic problems and longer hospitalization.[6] The endoscope-assisted transoral surgery can be used for selected well encapsulated PPS tumors with minimal complication. In this report, we present a case of giant pre-styloid PA in a male patient who underwent endoscope-assisted transoral surgery successfully without the need for transcervical incision or mandibulotomy. Case ReportA 67-year-old male referred to our clinic after incidental identification of oropharyngeal mass during his dental examination. He complained of worsening throat discomfort and foreign body sensation for a 4 months duration. He has diabetes mellitus and hypertension with the control of medication. Nasopharyngeal examination showed submucosal tumor on the right pharyngeal wall, extending from nasopharynx to the oropharynx, at that level medializing the soft palate and uvula. (Figure 1)
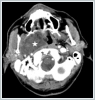
The surface mucosa of the tumor was normal. Computed Tomography (CT) showed large iso-dense mass at the right PSS displacing the major vessels posterolaterally. (Figure 2)
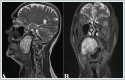
Magnetic Resonance Imaging (MRI) demonstrated huge mass in the right PPS, measuring 67mm x 37mm x 42 mm. (Figure 3)
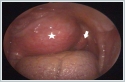
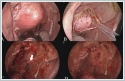
On MRI, the tumor showed a low signal on T1-weighted sequences, a high signal on T2-weighted sequences. There was no flow voids suggesting paraganglioma inside the lesion. Fine needle aspiration cytology (FNAC) revealed PA and did not suggest malignancy. As a result of the patient's assessment, it was decided to excise the tumor with an endoscope-assisted transoral approach. Under general anesthesia with nasal intubation, the Boyle-Davis mouth gag was applied for adequate exposure to his soft palate. The tumor was covered by the normal oral mucosa and medializing the uvula. (Figure 4A) After local anesthesia application, the incision was made using a monopolar diathermy. The tumor capsule was then visible following a blunt dissection with forceps. (Figure 4B) Using of angled and zero degree endoscope allowed a broad view of the lateral margin of the tumor. Following a careful dissection by combination of forceps, finger and diathermy the tumor was mobilized from its bed. The tumor was completely excised without capsule rupture or tumor fragmentation. The tumor bed inspected for any bleeding that was secured using bipolar diathermy. (Figure 4C) Oxidized cellulose sheets were used to achieve hemostasis. The incision was closed using 3/0 polyglycolic acid. (Figure 4D)
Histopathological examination showed that the tumor measured 60mm x 47mm x 39mm in size. The tumor was pathologically diagnosed as PA. The surgery was successfully done without using a cervical incision or mandibulotomy. A feeding nasogastric tube was inserted just after the operation. Nasogastric feeding was started after the surgery and it was removed at post-operative day 3. There were no complication observed and his swallowing and chewing were not affected. The patient was discharged from hospital at fourth post-operative day. A review at the third week after operation showed that the wound healed well. (Figure 5)
There has been no recurrence of tumor 1 year after surgery. DiscussionSymptoms of PPS tumors occur when they are larger than 2.5-3 cm due to the deep localization of this area.[7] For this reason, they are usually diagnosed by routine physical examination or on imaging performed for other purposes. In this case the mass was diagnosed upon dental examination incidentally. The preoperative diagnostic techniques are CT, MRI, angiography, FNAC and core biopsy. Imaging is crucial for the assessment of the tumors of PPS. Both MRI and CT are used to determine the origin of PPS tumors as the pre-styloid or the post-styloid. They also provide information about the extent of disease, type of tumor and relationships to parotid and the neurovascular structures. The displacement of the pre-styloid fat pad and carotid artery is important for the differential diagnosis of the tumors.[8] When carotid artery is displaced posteriorly, this suggests that tumor arises from the pre-styloid region. Post-styloid tumors generally displaced the artery anteromedially. As in this patient MRI scans show well circumscribed lesion in the prestyloid region. The tumor had low signal on T1-weighted sequences and high signal on T2-weighted images. It was consistent with a benign PA. Transoral FNAC is feasible diagnostic workup for PPS tumors however %25 of it may be nondiagnostic because of the adequate cellular material.[9] Transoral incisional biopsy should be avoided as opening the tumor capsule increases the risk of recurrence. Some authors have suggested that preoperative biopsy may not be necessary if imaging shows a benign tumor.[4,7] FNAC was performed to the patient and the result was compatible with PA. PA is benign, slowly growing salivary gland tumor. Most tumors originate in the superficial lobe of the parotid gland. PAs are rarely found in the PPS. The PA in the PPS, originates either from the parotid gland or from some aberrant minor salivary glands. Most of these tumors remain silent for a long time even if they displace, or in contact, vital structures located in the PPS.[10] The surgical excision of PPS tumors is the best treatment. However, surgical treatment of this tumors is challenging due to the deep localization of this space and its close relationship with vital structures in this region. Several surgical approaches have been described in the literature.[3,11,12,13] The choice of surgical approach depends on tumor location, size, histopathology and relation to the surrounding neurovascular structures. The main goals of the surgery are complete tumor excision under good exposure, protect vital structures and minimal functional and/or cosmetic adverse effects. Six main surgical approach have been described: transcervical, transparotid, mandibular split, transcervical-transmastoid, infratemporal fossa and transoral.[3] The most commonly used is the transcervical approach which provide adequate visualization. However external approaches have more complications, delay in return to normal nutrition and longer hospital stay.[6] Transoral approach initially described by Ehrlich and it was indicated for small, nonvascular tumors.[14] However this technique was not been adopted for many years due to poor exposure, inability to control bleeding, nerve injury, and incomplete removal. Recently, this situation has changed with the expansion of endoscopic devices and transoral robotic surgery. Using zero and angled endoscopes, allows visualization of hidden areas, enable to control bleeding and complete excision of the tumor. The improved exposure provided us to have low morbidity. However, endoscopic surgery requires a second hand that holds the endoscope during the surgeon dissects and sutures the tissue. Biggest advantage of transoral approach is that lack of any need for external incision. Additional advantages of this technique are short hospitalization, minimal blood loss and a most comfortable postoperative period. Therefore, we suggest the endoscope-assisted transoral surgery as an alternative when dealing with benign PPS tumors. References
Presented at15. Uluslararası Kulak Burun Boğaz ve Baş Boyun Cerrahisi Kongresi |
|||||||
| Keywords : parafaringeal tümör , pleomorfik adenom , tükürük bezi , endoskop yardımlı , transoral yaklaşım , prestiloid | |||||||
|